Research
PREDICT-Meso Network members carry out a range of basic and translational research studies to help improve our understanding of mesothelioma. These include genomics, epigenomics, transcriptomics, immune landscape, proteomics, breathomics, target drug validation and risk profiling.
As the Network progresses, we will post details of studies and projects, their results and impact, and how to get involved in mesothelioma research in the sections below:
Meso-ORIGINS
Mesothelioma Observational study of RIsk prediction and Generation of benign-meso tissue pairs, Including a Nested MRI Sub-study
Meso-ORIGINS forms the prospective tissue collection element of the PREDICT-Meso International Accelerator Network. This study received West of Scotland Research Ethics Committee approval in October 2021 (REC ref 21/WS/0120).
Our aim is to generate a large prospective cohort of asbestos-exposed patients with benign initial biopsies that we can retrieve and then match to any subsequent samples acquired at mesothelioma evolution. These samples will be used in downstream pre-clinical work packages to define the biology driving mesothelioma evolution, define new drug targets and validate these in a suite of pre-clinical models generated using the same material. By the end of the 8-year PREDICT-Meso program we aim to have drug-target combinations ready for human trials.

Arm A
Arm A will recruit asbestos-exposed participants with benign initial pleural biopsies. Study activities include:
- Retrieval of the initial benign pleural tissue biopsies
- 2 year follow up, with collection of repeat biopsies in patients who develop Pleural Mesothelioma (PM) during this period and a proportion of patients with at least 18 months of benign follow-up. Blood samples and associated imaging will also be collected.
- Baseline collection of blood and breath (+/-MRI in sites recruiting the sub-study (see below)). This data will be used for for risk profiling – helping us to design future studies of early intervention in those at high-risk of mesothelioma evolution
Arm A MRI Sub-Study
Perfusion MRI can identify the earliest stages of PM with >90% sensitivity and specificity, including in patients with only minimal pleural thickening. Patients will be recruited from within Arm A at centres participating in the MRI sub-study (estimated 6 sites).
Potentially eligible patients will be identified during the first visit in Arm A and invited back within 2 weeks for a contrast-enhanced MRI. This is a one-time additional visit. Participants will then continue on the Arm A pathway as above.
Arm B
A characteristic of PM is that multiple tumours can exist in different areas of pleural space and these may progress at different rates (spatial and temporal heterogeneity). In between there may be areas of benign pleural inflammation that does not progress to malignancy at all. Arm B will recruit patients with suspected but unconfirmed PM, allowing consent for multi-region dedicated research biopsies to be received prior to Thoracoscopy (LAT or VATS). These samples will carefully be carefully labelled and subjected to multiomic characterisation in order to better understand heterogeneity and its impact on subsequent progression and outcome.
Remote Observation
Patients who do not wish to take part Arm A follow up will be offered the option to consent to remote observation. This will allow the study team to remotely collect data and samples of blood, pleural fluid and pleural tissue that are taken as part of routine care but requires no active involvement from the patient, after the first visit.
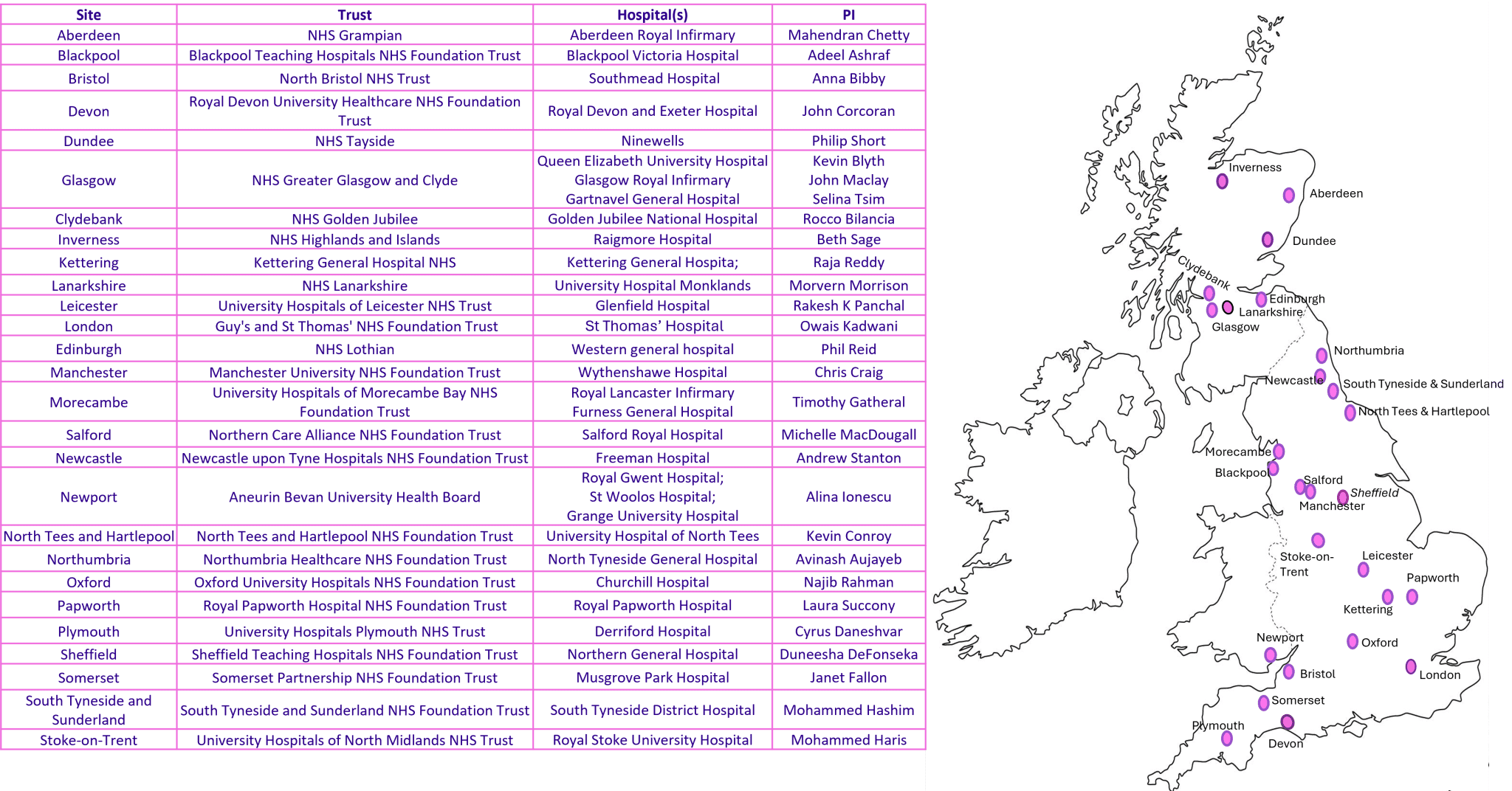
Meso-ORIGINS sites and PIs
Meso-ORIGINS
Get Involved
Meso-ORIGINS is an observational sampling study. Participants will be identified then followed up in parallel to routine clinical visits to see if there has been any evidence of mesothelioma evolution. The study has 2 arms (A and B). Arm A also includes a MRI sub-study and a remote observation option.
Arm A
Arm A will recruit asbestos-exposed participants with evidence of associated benign pleural disease based either on an initial pleural biopsy (of any form) or imaging within the last 1 year. Participants will be identified at outpatient clinic appointments, inpatient reviews, MDTs or via Arm B (see below).
A member of the research team will approach the participant, explain the study and provide a Participant Information Sheet (PIS) and consent form. Once consent has been accepted, participants will have baseline data recorded, their initial biopsies banked and non-invasive risk profiling tests performed. These will include a blood sample, an exhaled breath sample (+/- an MRI scan in participating centres, see MRI sub-study detail below). Pleural fluid samples will also be collected if patient has an indwelling pleural catheter (IPC) in-situ – it is acknowledged that not all patients will have an IPC.
Participants will then have regular study follow-up visit (every 6 months) for 2-years, ideally combined with routine clinical follow- up. A contrast- enhanced CT of the thorax should occur within 1-month of each follow-up visit, at which time blood sampling and follow-up data will be collected. Participants with clinical suspicion of mesotheliom at any time will have a repeat biopsy in clinical care- Meso-ORIGINS will collect these biopsies also. Participation in Arm A will end after 2-years follow up.
Arm A MRI Sub-Study
Potentially eligible patients will be identified during the first visit in Arm A, at which time an additional MRI sub-study PIS will be provided. Documentation of additional consent on a separate form is required as this is an additional voluntary element. The participant will be invited back within 2 weeks for a contrast-enhanced MRI in addition to completing a MRI safety questionnaire. This is a one-time additional visit. Participants will then continue on the Arm A pathway as above.
Arm B
Arm B will recruit eligible participants with suspected but unconfirmed MPM, prior to biopsy by thoracoscopy. Participants will be identified via urgent suspicion of cancer (USOC)/2-week wait urgent referral pathways. A member of the research team will approach the participant, explain the study, and provide a PIS and consent from. Following receipt of consent, blood and breath samples will be taken and thoracoscopy will be arranged – both LAT and VATS are acceptable in this arm. At LAT or VATS, multi-region research biopsies will be taken and carefully labelled. Pleural fluid will also be collected alongside clinically indicated diagnostic samples.
The participant will return within 2 weeks for clinical review and biopsy results discussion (Visit B3). This ends active participation in Arm B, however participants will still be followed up for 1 year to determine status (Visit B4). There are a number of ways this follow up may occur:
1) Participants with benign pleural biopsies may be directed to the Arm A, although this is not mandatory. Note that a separate consent process for Arm a will be required.
2) Participants with benign pleural biopsies who chose not to take part in Arm A, will be remotely followed up to check status at 1 year as part of continued Arm B participation.
3) If pleural mesothelioma is confirmed participants will be remotely followed up to check status at 1 year as part of continued Arm B participation. They may be directed to the ASSESS-Meso study (a sister study within PREDICT-Meso) if this is open at site. ASSESS-Meso is a longitudinal observational study following patients with Mesothelioma (REC approval 17/SW/0019). More details can be found here. ASSES-meso
4) Any other diagnosis, particpants exit study after visit B3.
Remote Observation
Patients who do not wish to take part in Arm A follow up will be offered the option to consent to remote observation. This will allow the study team to remotely collect follow up data and surplus of samples of blood, pleural fluid and pleural biopsies that are taken within routine care, but requires no active involvement from the patient. Participation in remote observation will end after 2 years follow up.
ASSOCIATE PRINCIPAL INVESTIGATOR (PI) SCHEME
We are delighted to announce that PREDICT-Meso Clinical study Meso-ORIGINS is a now registered study in the NIHR Associate Principal Investigator (PI) Scheme.
The Associate PI Scheme is a six month in-work training opportunity, providing practical experience for healthcare professionals starting their research career.
People who would not normally have the opportunity to take part in clinical research in their day-to-day role have the chance to experience what it means to work on and deliver an NIHR portfolio trial under the mentorship of an enthusiastic Local Principal Investigator (PI). A Principal Investigator is an individual responsible for the conduct of a research study at a site.
Associate Principal Investigators receive formal recognition of engagement in NIHR Portfolio research studies through the certification of Associate PI status, endorsed by the NIHR and Royal Colleges.
Check out the video below for more information
The NIHR website has a wealth of further information including:
How to apply https://www.nihr.ac.uk/health-and-care-professionals/training/applying-to-be-an-associate-principal-investigator.htm
Associate PI Toolkit https://sites.google.com/nihr.ac.uk/associatepischeme/toolkits/associate-pi-toolkit
If you are interested in this unique training opportunity and want to be an Associate PI for Meso-ORIGINS, please contact us at PREDICT-Meso@glasgow.ac.uk for information on the Meso-ORIGINS study, local sites and PIs. Looking forward to having you on board!
Meso-ORIGINS can now be found on the CRUK website under their 'find a clinical trial' search for those interested in Mesothelioma
Click the button below to go to this page and view an easy to read lay summary of the Meso-ORIGINS study